
주문제작 서비스 문의
찾으시는 제품이 저희 사이트에 없으신가요? 단백질 주문 제작 서비스를 이용해보세요!
주문제작 서비스 문의 >>
























 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
| 제품번호 | 종 | 제품 설명 | 구조 | 순도 | 특징 |
|---|---|---|---|---|---|
| GLN-N52H3 | Nipah virus | Nipah virus Glycoprotein G, His Tag (MALS verified) |  |
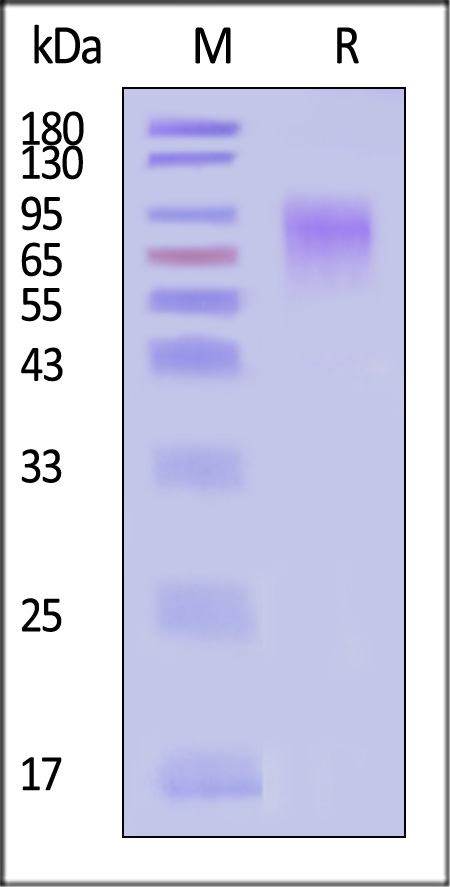
|
|
| GLN-H52H3 | Hendra virus | Hendra virus Glycoprotein, His Tag (MALS verified) |  |
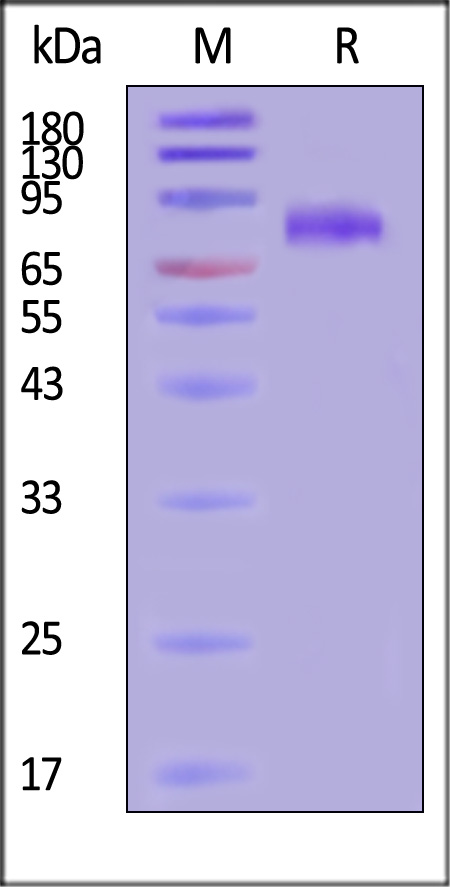
|
Anti-Nipah/Hendra Glycoprotein G Antibody, Human IgG1 captured on Protein A Chip can bind Nipah virus Glycoprotein G, His Tag (Cat. No. GLN-N52H3) with an affinity constant of 0.188 nM as determined in a SPR assay (Biacore 8K) (Routinely tested).
This web search service is supported by Google Inc.






